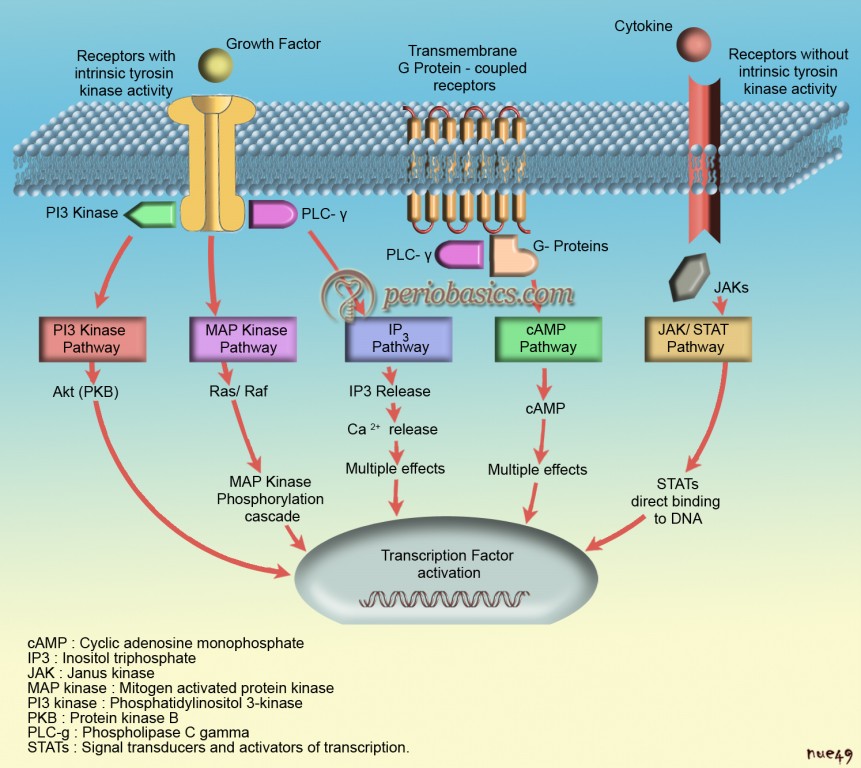
Introduction to growth factors
Growth factors are polypeptide molecules released by cells in the inflamed area that regulate events in wound healing. These are naturally occurring proteins that regulate various aspects of cell growth and development, acting locally or systemically. “Growth factor” is a general term to denote a class of polypeptide hormones that stimulate a wide variety of cellular events such as proliferation, chemotaxis, differentiation and production of extracellular matrix proteins. Initially, growth factors were described as soluble molecules, but with present evidence it is clear that the binding of growth factors to the extracellular matrix (ECM) is a major mechanism which regulates growth factor activity.
Growth factors are proteins that may act locally or systemically to affect the growth and function of cells in several ways. Exogenous growth factors can be used to supplement natural growth factors in wound healing which serves as basis for many upcoming regenerative therapies. In the following discussion, we shall study in detail about various growth factors, their sources and target cells and their effects at cellular and molecular level.
Rationale for using growth factors in periodontal regeneration
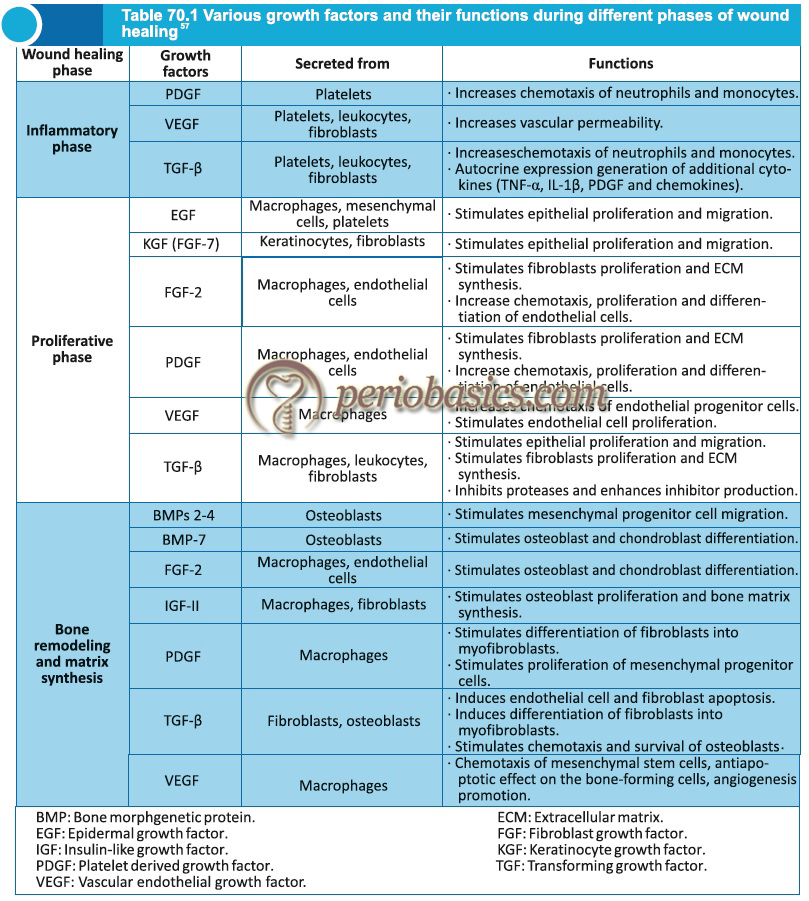
In general when injury occurs, a “well-orchestrated” cell-cell and cell-ECM interaction is initiated which begins the healing process. A complex activity of various molecules including cytokines and growth factors begins in the inflamed area initiating ECM remodeling. Tissue repair studies conducted on animals provide evidence that key growth factors involved in wound healing include EGF, TGF-α and β, PDGF, acidic and basic FGF.
Various studies have shown that if one or more of the above chemical mediators are removed from the healing site, the process of healing is hampered. Every growth factor has specific role during development and healing, and identification of their exact mechanism of action and their affects on various cells is …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Classification of growth factors
Growth factors and cytokines have historically been classified into ‘families’ based on their source cell type and apparent activity and/or impact on a given cell type, system, or tissue. Following is the description of various growth factors belonging to various growth factor families.
Platelet-derived growth factor family: PDGF-AA, PDGF-BB, PDGF-AB, PDGF-CC and PDGF-DD.
Vascular endothelial growth factor family: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and Placenta-derived growth factor (PlGF).
Transforming growth factor-beta superfamily: TGF-β, Inhibins, Activin, Anti-müllerian hormone, Bone morphogenetic proteins, Decapentaplegic and Vg-1.
Epidermal growth factor family: Epidermal Growth Factor, TGF-α, Schwannoma-derived growth factor, Heparin-binding EGF (HB-EGF), Betacellulin, Epiregulin, and Neuregulin (NRG) family.
Fibroblast growth factor family: Acidic FGF (aFGF/FGF-1), Basic FGF (bFGF/FGF-2), Int-2 (FGF-3), hst/KS3 (FGF-4), FGF-5, FGF-6, Keratinocyte growth factor (FGF-7), Androgen-induced growth factor (AIGF or FGF-8), Glia activating factor (GAF or FGF-9), Keratinocyte growth factor-2 (FGF-10), FGFs 11-14, FGF-15, FGFs 16-19, FGF-20 (XFGF-20), and FGFs 21-23.
Insulin family: Insulin-like growth factors I (IGF-I) and Insulin-like growth factors II (IGF-II).
Hepatocyte growth factor family: Hepatocyte growth factor (HGF), Macrophage-stimulating protein (MSP).
Colony-stimulating factors (CSF): These are the molecules which have the ability to induce the development of distinct cell lineages e.g. IL-3, Macrophage-CSF (M-CSF) and Granulocyte-CSF (G-CSF), Erythropoietin.
Neurotrophin Family: Neurotrophic factor, Brain-derived neurotrophic factor (BDNF), Neurotrophin-3 (NT-3), NT-4, NT-5, and NT-6.
Evidence for role of growth factors in periodontal regeneration
There is a lot of evidence available which shows regenerative potential of growth factors in periodontal regeneration. Local administration of platelet-derived growth factor (PDGF) to periodontal osseous defects leads to significant regeneration of bone, cementum, and periodontal ligament 2. In a dog study, Cho et al. (1995) 3 demonstrated that PDGF application with guided tissue regenerative therapy in critical size bone defects resulted in complete bone regeneration. Similar results were demonstrated by Park et al. (1995) 4 in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor.
Periodontal ligament (PDL) cell proliferation and migration during healing is considered to be the major event during periodontal regeneration. It has been suggests that the polypeptide growth factors including PDGF, IL-1, and TGF-β are the mediators of these cellular events in wound healing. One study investigated the effects of these growth factors on human PDL cell mitogenesis, and the regulatory influences of TGF-β on the response to PDGF and IL-1. Results of the study demonstrated that PDGF-AA and PDGF-BB are major mitogens for human PDL cells in vitro, and supports the role for TGF-β as a regulator of the mitogenic response to PDGF in these cells 5. In a comparative study, response of PDL cells and gingival fibroblasts to polypeptide growth factors was investigated. The migratory responses of PDL cells and gingival fibroblasts to …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
In one study, a combination of PDGF and IGF-I was used in periodontitis-affected teeth in beagle dogs. The results showed that a continuous layer of osteoblasts lined the newly formed bone, and there was a dense cellular “front” at the coronal extent of the new bone. The results of the study suggested that application of the combination of PDGF and IGF-I may enhance regeneration of the periodontal structures 7. A clinical trial evaluated a combination of recombinant human platelet-derived growth factor-BB and recombinant human IGF-I in periodontal bone regeneration in patients with periodontal disease. An increase of 2.08 mm of new vertical bone height and 42.3% osseous defect fill in the human-PDGF/IGF-I subjects was observed as compared to controls who demonstrated only 0.75 mm and 18.5% gains in new bone height and osseous fill, respectively 8.
It has been shown that fibroblast growth factors (FGFs) are strongly mitogenic to bone marrow stromal cells and are able to maintain the self-renewal of these cells in culture 9. FGF-1 and FGF-2, in vitro, stimulate osteoblast …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
Platelet-derived growth factor (PDGF)
PDGF was originally purified from human platelets. Kohler and Lipton (1974) 12 and Ross et al. (1974) 13 discovered that the bioactive mediators released from platelets are the principal source of mitogenic activity present in serum, and are responsible for the growth of many cells in culture that are serum dependent. PDGF has been found to be produced by various other cells, for example, monocytes, megakaryocytes, vascular endothelium, smooth muscle cells, and transformed cells 14, 15. PDGF was shown to stimulate wound healing, which resulted in its first therapeutic application, becaplermin (Regranex®), a gel containing recombinant PDGF-B, which accelerates ulcer repair. PDGF was the first growth factor to be evaluated in preclinical periodontal and peri-implant regenerative studies.
PDGF is a well-characterized regulatory protein with an isoelectric point of 9.8 and a molecular weight of approximately 30,000 Da 16. The structure of the molecules has been described as two disulfide-bonded polypeptide chains that are encoded by two different genes, PDGF-A and PDGF-B, located on chromosomes 7 and 22, respectively 1, 17. Two new forms, PDGF-C and PGDF-D have been described recently 18. So, PDGF has 5 isoforms, PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC and PDGF-DD. Among these, 3 isoforms PDGF-AA, PDGF-AB and PDGF-BB have been extensively studied 2, 7, 19-22.
Platelets synthesize a mixture of the three possible PDGF isoforms (70% AB, 20% BB, 10% AA) 23. They differ in their functional properties as well as in their secretory behaviors. PDGF-AA and PDGF-AB are rapidly secreted from the producer cell, whereas PDGF-BB remains to a large extent associated with the producer cell and relatively small amounts are secreted 24. PDGF acts on the target cells by binding to α and β receptors on their cell surfaces and in turn …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ……...
Actions of PDGF:
PDGF is a chemoattractant for fibroblasts, leukocytes and smooth muscle cells. It acts synergistically with IGF-I, promoting protein synthesis and production of ECM. It has mitogenic effects on osteogenic cells, promoting their proliferation and migration in the healing area. It also promotes synthesis of fibronectin and collagen Type I, III and V. It inhibits collagenase and plasminogen activator. PDGF up-regulates the expression of angiogenic molecules like vascular endothelial growth factor (VEGF) and hepatocyte growth factor, and also the proinflammatory cytokine interleukin-6; thereby indirectly promoting periodontal regeneration. The recombinant human PDGF-BB (GEM2 IS) has received FDA clearance for use. The vehicle used for GEM2 IS is tricalcium phosphate which provides appropriate localized concentration of PDGF at the wound site for sufficient period of time, facilitating its desired affects during healing.
Literature review:
In vitro and in vivo studies have demonstrated that PDGF is a potent chemotactic and mitogenic factor for gingival and PDL fibroblasts, cementoblasts and osteoblasts 7, 25-27. It has been demonstrated that PDGF-BB applied to root surfaces increased proliferation period of PDL cells, cementoblasts, osteoblasts, perivascular cells and endothelial cells 28.
Lynch et al. (1991) 29 treated 13 dogs with human recombinant (rh) PDGF-BB and IGF-I in methyl cellulose gel. Five weeks after surgery, histological analysis demonstrated a significant increase in new bone and cementum formation in the growth factor treated sites over that in control sites. In another study, the effect of PDGF and IGF-I was evaluated on periodontal regeneration in vivo, in a non human primate model. The results of the study …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
In an implant study on dog model, direct application of an rh PDGF/IGF mixture into implant sites produced a two to three times increase in the number of peri-implant spaces filled with bone as compared to initial readings 29. Another study demonstrated that bone density and bone-to-implant contact were increased by two-fold for the growth factor treated sites, as compared to the membrane alone or membranes combined with bone grafts 31.
The application of PDGF in horizontal ridge augmentation procedures was evaluated in one study. Bilateral mandibular surgically created defects were treated with either beta-tricalcium phosphate (β-TCP) covered with a collagen membrane (CM) or a combination of β-TCP + CM + rhPDGF-BB, following a split-mouth design. The results of the study demonstrated that the group containing rhPDGF-BB showed better results in terms of mineralized tissue and total augmented area at 3 weeks 32. Encouraging results have been demonstrated for maxillary sinus floor augmentation procedure, using anorganic bovine bone mineral combined with rhPDGF-BB 33.
Transforming growth factor (TGF)
Transforming growth factor-α (TGF-α):
It belongs to the epidermal growth factor (EGF) family of cytokines. It is a mitogenic polypeptide and secreted protein, which is expressed by monocytes, keratinocytes, and various tumor cells. It has 80% homology with EGF and it binds to the cellular EGF receptor. EGF and TGF-α are equipotent at inducing in vitro endothelial cell proliferation and bind equally well to endothelial cell EGF receptor. It acts synergistically with TGF-β to stimulate anchorage-independent cell proliferation and produce a mitogenic response.
Transforming growth factor-β (TGF-β):
TGF-β belongs to TGF-β superfamily, which has many multifunctional structurally related growth and differentiation factors associated to the inflammatory response. These factors play an important role in apoptosis, angiogenesis, wound healing and fibrosis. TGF-β is a highly conserved dimeric polypeptide with a molecular weight of 2500 Da and consists of 2 amino acid chains linked together by disulfide bonds. It is found in highest concentration in bone and platelets. TGF-β is encoded by three different genes TGF-β1, TGF-β2, and TGF-β3. TGF-β1 contains 390 amino acids and TGF-β2 and TGF-β3 each contain 412 amino acids. TGF-β has been shown to increase the biosynthesis of collagen Type I, fibronectin and osteocalcin, as well as bone matrix …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
It can also modulate other growth factors such as PDGF, TGF-α, EGF and FGF possibly by altering their cellular response or by inducing their expression. TGF-β has a marked effect on ECM homeostasis, being an important mediator of fibroblast proliferation and ECM synthesis. It stimulates mesenchymal cells and inhibits epithelial cell proliferation. TGF-β1 has pro-fibrotic role which can be related to its role in gingival overgrowth. During the healing process, it promotes collagen fiber deposition and causes fibrosis which can be related to gingival enlargement during inflammation. The precise role of TGF-β1 in the pathogenesis of periodontitis-induced gingival overgrowth is still unclear.
Actions of TGF-β:
Briefly, primary actions of TGF-β are,
- It acts as an important factor for fibroblast migration and proliferation.
- It has pleiotropic effects on cell proliferation, which can either stimulate or inhibit proliferation in different cell types and within the same cell type.
- It promotes synthesis of collagenous matrix and regulates extracellular matrix.
- A weak mitogen for osteoblastic cells.
- It may play an important role in immune regulation.
Literature review:
TGF-β plays a significant role in periodontal regeneration. However, very few periodontal regeneration studies using TGF-β have been performed. The initial animal experiments done on dogs 34, 35 and sheeps 36 to evaluate the regenerative potential of TGF-β1 gave disappointing results. Other animal experiments demonstrated increased amount of bone healing adjacent to dental implants 37. TGF-β has synergistic effect with PDGF in stimulating gingival fibroblast and PDL cell proliferation. A study done to measure the time-sequence response of RNA and protein synthesis …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
Bone morphogenetic proteins (BMPs)
BMPs belong to the transforming growth factor-β (TGF-β) superfamily, which consists of a group of related peptide growth factors. They have numerous cellular functions including development, morphogenesis, cell proliferation, apoptosis, and ECM synthesis. These proteins are synthesized as large precursor molecules which after dimerization are cleaved proteolytically at a consensus Arg-X-X-Arg site to generate mature dimers. Presently, more that 20 BMPs have been identified which have been studied for their biological activity. Extensive research has been done on these proteins and these may be potential candidates for newer periodontal regenerative techniques. A detailed description of BMPs is available in “Bone morphogenetic proteins in periodontal regeneration”.
Fibroblast growth factors (FGF)
These are family of structurally related strongly heparin-binding peptides that have been implicated in healing and regeneration. To date, 23 distinct FGFs have been discovered, numbered consecutively from 1 to 23. All the FGFs have a central core of 140 amino acids that is highly homologous between different family members. The two best studied members, acidic FGF (a FGF) and basic FGF (b FGF), interact with the same cell surface receptor and thus share similar biologic activity, although b FGF usually exhibits 30- to 100-fold greater potency in vitro. Both FGF-1 and FGF-2 were initially isolated from bovine pituitary extracts based on their stimulation of [3H] thymidine incorporation in 3T3 fibroblasts 39, 40. They have been shown to stimulate mitogenesis and chemotaxis in PDL cells 41, 42. To mediate their range of effects, FGF proteins signal via membrane-spanning tyrosine kinases (FGFR) and there are a wide variety of mechanisms for receptor regulation and availability. Atleast 4 FGF receptors have been …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
Fibroblast growth factor-1 (FGF-1)/acidic FGF (a FGF):
FGF-1 has an isoelectric point range of 5.6-6.0 and a molecular weight of approximately 15,000 Da. It is a 155 amino acid protein. This protein functions as a modifier of endothelial cell migration and proliferation, as well as an angiogenic factor. It acts as a mitogen for a variety of cells. FGF-1 is considered to function in several important physiological and pathological processes, such as embryonic development, morphogenesis, angiogenesis and wound healing.
Fibroblast growth factor-2 (FGF-2)/ basic FGF (b FGF):
FGF-2 has an isoelectric point of approximately 9.6 and a molecular weight in the range of 16,000-18,000 Da. Human FGF-2 occurs in low molecular weight (LMW) and high molecular weight (HMW) isoforms. LMW FGF-2 is primarily cytoplasmic and functions in an autocrine manner, whereas HMW FGF-2 is nuclear and exert activities through an intracrine mechanism 43. Immunohistochemical analysis of tissues for FGF-2 often reveals FGF-2 in association with the ECM and in basement membranes attached to heparan sulfate. The binding of FGF-2 to the FGF receptor (FGFR) activates a signal transduction cascade, eventually stimulating cell proliferation and differentiation. This binding of FGFs to their tyrosine kinase-signaling …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
In periodontium, FGF-2 is present in the ECM, as well as in the cementum and can function as a local factor at the site 46. In inflamed periodontal tissues, FGF-2 has been identified in the gingival epithelium, inflammatory cells and connective tissue 47.
Actions of FGF:
Briefly, actions of FGFs are as follows,
At cellular level:
- FGFs are considered to be competence growth factors. A competence growth factor is the one which stimulates resting cells in G0 phase to enter the cell cycle in G1 phase.
- They have been associated with increased mitogenesis of cells.
- It is found in association with the ECM in the basement membranes and is attached to heparan sulphate, which provides protection from degradation and allows it to maintain its biological potential.
During wound healing:
- They play an important role during wound healing. The FGF-1, FGF-2 and keratronocyte growth factor (KGF) are primary FGFs involved in wound healing.
- They stimulate proliferation of most of the major cell types involved in wound healing, including vascular endothelial cells, fibroblasts, keratinocytes and chondrocytes.
- FGF-2 also stimulates epithelialization, fibronectin, proteoglycan and collagen synthesis.
- It has been shown that administration of FGF-2 at the time of wound closure not only significantly increases the breaking strength of the wound but also improves the quality of the scar 18.
- FGF-2 stimulates periosteum derived cells in early stages of bone healing.
Angiogenesis:
- FGF-2, in particular has the ability to induce all steps necessary for new blood vessel formation both in vivo and in vitro 18. It regulates the production of collagen Type I and laminin by PDL cells. Laminin is one of the most important biological substances which is involved in angiogenesis 41.
- FGF-1 stimulates endothelial cell proliferation which enhances new blood vessel proliferation in the healing area.
Effect on PDL cells:
- They have chemotactic and mitogenic effects on PDL cells.
- Due to their overall effects, they play a vital role in the process of periodontal regeneration.
Literature review:
The effect of FGFs on osteoblasts has been the focus of research. It has been shown that FGF-1 and FGF-2 stimulate cell proliferation but inhibit alkaline phosphatase activity and reduce collagen Type I and osteocalcin expression 10, 11, 45, 49-51. Initial research demonstrated that FGF-2 and FGF-1 are stored in bone matrix and may be the important factors in regulating osteoblastic cells 52. In one study, the effects of recombinant human FGF-2 (rhFGF-2) on human neonatal calvaria osteoblastic cells was evaluated. The results of the study showed that the effects of FGF-2 on osteoblast differentiation are …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
The expression of receptors for FGF-2 on human PDL cells was investigated in one study which concluded that the responsiveness of PDL cells to FGF-2 is altered during the course of their culture due to change in the density of receptor on their surface. So, the density of FGFR expression might be a marker for the cytodifferentiation of PDL cells into mineralized tissue forming cells 54.
One study investigated the role of FGF-2 in the wound healing and regeneration of periodontal tissues in surgically created 2-wall, 3-wall and furcation class II bone defects in beagle dogs and primates. Results of the study demonstrated that FGF-2 enhances the proliferative responses of human PDL cells, which express FGF receptor-1 and -2, but inhibits the induction of alkaline phosphatase activity and mineralized nodule formation by PDL cells. Hence, FGF-2 helps in regeneration of periodontal structures by inducing proliferation of PDL cells 55.
In a double-blind controlled trial, the efficacy of the local application of rhFGF-2 in periodontal regeneration was investigated. The trial included 253 adult patients with periodontitis. Modified Widman flap surgery was performed, during which 200 μL of the investigational formulation containing 0% (vehicle alone), 0.2%, 0.3%, or 0.4% FGF-2 was administered to 2-or 3-walled vertical bone defects. Sites with FGF-2 application …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
Insulin-like growth factors (IGFs)
IGFs were first described in 1957 by Salmon and Daughaday 58. They are a family of mitogenic proteins that control growth, differentiation, and maintenance of differentiated function, in numerous tissues. These growth factors are single-chain proteins that share 49% homology with pro-insulin 16. The IGF family includes three ligands (insulin, IGF-I, and IGF-II), their corresponding cell surface receptors (IR, IGF-IR, and IGF-IIR), and at least six IGF-binding proteins (IGFBPs). The six identified forms of IGFBPs are synthesized and secreted by a variety of cell types 59.
Two well-described members of this group are IGF-I and IGF-II which are similar in structure and function but are regulated independently 60. Both IGF-I and IGF-II are anabolic peptides with 65% amino acid sequence homology and similar biologic activities. However, synthesis of these factors is regulated and controlled in different manner 1. The precursor molecules of IGF-I and IGF-II have 195 and 156 …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
Insulin-like growth factor-I (IGF-I):
IGF-I is a 70-amino-acid protein with a molecular weight of 7649 Da and an isoelectric point of 8.4. It has endocrine, paracrine, and autocrine effects. It is mainly produced by the liver but virtually every tissue is able to secrete IGF-I for autocrine/paracrine purposes 61. It shares >60% homology with IGF-II and around 50% homology with proinsulin structures 62. Although, IGF-I binds to six forms of high affinity IGF binding proteins (IGFBPs 1 to 6), which either promote or inhibit its actions; but majority of IGF-I actions are mediated through the union of IGF-I to its putative receptor, IGF-IR, a tyrosine kinase.
Actions of IGF-I:
Growth and development:
- It plays a very important role in fetal growth and differentiation.
- It has important role during central nervous system development, where it acts as a neuroprotector. It may promote proliferation and/or survival of oligodendrocytes and their precursors.
- It has an important role in the cardiovascular system. It has been shown that IGF-I and its receptor are expressed in the myocardium and both aortic smooth muscle and endothelial cells 63, 64.
- IGF-I plays important roles in T-lymphocytes development and function. Specifically, it can increase the number of CD4+ CD8+ immature T-cells in rat thymus and spleen 65, promotes T-cell survival 66, proliferation, chemotaxis and maturation, and blocks spontaneous and induced programmed cell death 67.
- IGF-I has been shown to play an important role in liver regeneration. During liver regeneration, along with IL-6, TNF-α, HGF and TGF-α/EGF, it supports hepatocyte proliferation and accelerates DNA synthesis 68, 69.
Effects at cellular level:
- The major effects of IGF-I on cells are,
- It is a powerful chemoattractant of fibroblasts and it leads to the periodontal regeneration by stimulating the formation of mesenchymal tissues including collagen, bone and cementum 70.
- It upregulates cementoblast mitogenesis, phenotypic gene expression, and mineralization 71.
- IGF-I has demonstrated a capacity to increase bone cell mitoses and increase the deposition of matrix. PDGF and IGF have shown the ability to work together during the reparative stages of bone healing 2, 72.
Role in wound healing:
Present evidence suggests that IGF-I is an important factor involved during wound healing because it is mitogenic for keratinocytes 75 and is a potent chemotactic agent for vascular endothelial cells. IGF-I expression is modulated during wound healing. Its levels are increased in healing site suggesting that its presence is requires for adequate wound healing 76, 77.
Effect on periodontal ligament cells:
In general, IGF has been shown to increase fibroblast proliferation. One study demonstrated the mitogenic effects of IGF-I on PDL fibroblastic cells and concluded that a synergistic effect results from using a combination of PDGF-AB and IGF-I 78. Similarly, another study showed that IGF-I can stimulate the synthesis of DNA in PDL fibroblasts, likely via binding to high-affinity cell surface receptors 79.
Insulin-like growth factor-II (IGF-II):
IGF-II [also known as multiplication stimulating activity (MSA)] is a 67-amino-acid neutral peptide with a molecular weight of 7471 Da. IGF-II binds to IGF-II receptor (IGF-IIR), to IGF-IR and weakly to the insulin receptor (IR). It is not as potent as IGF-I. There is relatively less clinical research data available of IGF-II in relation to periodontal regeneration. The effect of IGF-II on the metabolism of gingival fibroblasts is still uncertain.
Epidermal growth factor (EGF)
The EGF is a multifunctional cytokine involved in a variety of functions, including epithelial growth and differentiation, and wound healing. In 1986, Stanley Cohen received the Nobel Prize for his work elucidating the role of the EGF in the regulation of cell growth and development 80. The EGF …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
The EGF is a small protein comprising of 53 amino acids. It is produced by epithelial cells, fibroblasts and many other cell types. It is found in membrane-associated and soluble forms. The low molecular weight soluble EGF is generated through proteolysis of a large ~130,000 molecular weight transmembrane precursor. Both soluble and membrane-associated forms of EGF are active. Its actions are activated with its attachment to the epidermal growth factor receptor (EGF-R). This receptor induces cell differentiation and proliferation upon activation through the binding of one of its ligands. The EGF-R has 3 major regions:
1. Extracellular domain which contains growth factor.
2. Hydrophobic transmembrane domain
3. Cytoplasmic domain which contains tyrosine specific protein kinase.
Literature review:
In a study, the expression of EGF in normal and surgically wounded PDL tissues and its effect on chemotaxis and expression of osteoinductive and angiogenic factors in human PDL cells (HPDLCs) was investigated. It was observed that the levels of EGF and IL-1β were found to be upregulated in a PDL tissue-injured model of rat. The results of the study indicated that EGF is upregulated under inflammatory conditions which plays roles in the repair of wounded PDL tissue 81.
A study was done to understand the role of EGF-R in PDL fibroblasts on rat derived PDL cells and and ROS 17/2.8 cells (highly differentiated osteoblastic osteosarcoma cells). These cells were cultured and treated with TGF-α, EGF, dexamethasone or a combination of EGF and dexamethasone. Alkaline phosphatase activity was then calculated as an indicator of mineralized tissue formation. It was observed that PDL fibroblastic cells express numerous EGF-R, but their number decreases during their differentiation into mineralized tissue-forming cells, indicating that EGF-R may function in the stabilization of phenotype in PDL fibroblastic cells 82. Another study supported these findings 83.
Although, a lot of research work is going on EGF, but the effect of EGF on periodontal wound healing in vivo remains to be investigated.
Vascular endothelial growth factor (VEGF)
VEGF is a potent angiogenic factor and was first described as an essential growth factor for vascular endothelial cells. It is also known as vascular permeability factor (VPF). Originally, it was described as endothelial cell-specific mitogen 84.
Sources:
Macrophages.
Platelets.
Keratinocytes.
Tumor cells.
VEGF is an important growth factor involved in functions such as bone formation 85, hematopoiesis 86, wound healing 87, and development 88. The VEGF family comprises seven members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF (placenta growth factor). All members have a common VEGF homology domain.
Actions:
1. VEGF-A induces angiogenesis by following mechanisms,
a. increased migration of endothelial cells.
b. increased mitosis of endothelial cells.
c. increased matrix metalloproteinase activity.
d. increased αvβ3 activity.
e. creation of blood vessel lumen.
f. creates fenestrations.
2. VEGF-A is chemotactic for macrophages and granulocytes.
3. VEGF-A is involved in vasodilation (indirectly by NO release).
4. VEGF-B is involved in embryonic angiogenesis.
5. VEGF-D is needed for the development of lymphatic vasculature.
6. PIGF is important for vasculogenesis.
Literature review:
Studies have been done to investigate the role of VEGF in periodontal health and disease. One study investigated the presence of VEGF in human periodontal tissue and gingival crevicular fluid (GCF) in periodontal health and disease. Levels of VEGF in GCF and saliva of diseased as well as healthy controls were measured. VEGF in tissue was localized by immunohistochemistry. This study reported that VEGF could be relevant to angiogenic processes in healthy as well as diseased periodontal tissue and that the periodontal status influences the salivary level of VEGF 89.
Another study investigated the role of VEGF in periodontal disease progression and the effect of periodontal therapy on VEGF concentrations in GCF. Study included healthy (group 1), gingivitis (group 2), and chronic periodontitis (group 3) groups. A fourth group consisted of subjects from …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
Few studies have been done to evaluate the effects of VEGF on PDL cell behavior. One study investigated whether FGF-2 could induce VEGF-A expression in PDL cells and whether cell-to-cell interactions between PDL cells and endothelial cells could stimulate angiogenesis. The study concluded that FGF-2 induced VEGF-A expression in PDL cells and induced angiogenesis in combination with VEGF-A. The cell-to-cell interactions with PDL cells also facilitate angiogenesis 91.
Another study evaluate the effects of basic FGF-2 and VEGF on the proliferation, migration, and adhesion of human PDL stem cells in vitro. Human PDL stem cells were cultured in vitro using tissue culture method and were incubated with various concentrations of FGF-2 and VEGF. Study concluded that both FGF-2 and VEGF could simulate the proliferation of PDL stem cells in a dose dependent manner, and they had a synergistic effect. FGF-2 was more effective to promote the adhesive capacity of PDL stem cells as compared to VEGF. VEGF could facilitate the migration of PDL stem cells to the wound side 92.
Colony-stimulating factors (CSF)
The CSFs act on the stem cells, leading to lineage-specific differentiation. Stem cell factor (SCF) regulates differentiation of CD34+ stem cells whilst other factors modulate the synthesis of more specific cell types: erythropoietin (EPO) for red cells; granulocyte colony-stimulating factor (G- CSF) for neutrophils; granulocyte-macrophage colony-stimulating factor (GM-CSF) for macrophages and neutrophils; macrophage colony-stimulating factor (M-CSF, also called colony-stimulating factor-1 (CSF-1)) for …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book …….. Contents available in the book ………
Conclusion
In the above discussion, we discussed various aspects of growth factors and their present research in periodontal regeneration. As discussed in previous sections, various studies have shown that growth factors may effectively promote periodontal regeneration. But, there are multiple problems associated with using growth factors for periodontal regeneration, mainly including,
- Need for high local concentration.
- Non-specific activity on different cell lineages in time and space.
- Rapid loss of topically applied growth factors.
We are now working on the development of techniques which may address these problems and provide us an effective mechanism of delivering these growth factors in the area of periodontal regeneration. A detailed description of these techniques is available in chapter 73, “Tissue engineering in periodontics”.
References
References are available in the hard-copy of the website.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.